-
 Screening of Colorectal Cancer in TaiwanHan-Mo Chiu, M.D., Ph.D.
Screening of Colorectal Cancer in TaiwanHan-Mo Chiu, M.D., Ph.D.Clinical Professor, Department of Internal Medicine, National Taiwan University Hospital
Principal investigator, Taiwan Colorectal Cancer Screening ProgramGlobally, colorectal cancer (CRC) ranks third (1,849,518 new cases, 10.2% of total) as the most commonly diagnosed cancer after lung and breast, with it being second in women and third in men in 2018. It is also the second oncological cause of death worldwide, although with some global geographic differences in both incidence and mortality rates, with Asia contributing the highest, 957,896 (51.8%) of incident cases and 461,422 (52.4%) of deaths (all genders and ages) per 100,000 population in the world. Fecal immunochemical test (FIT) is a stool test that detects human hemoglobin in the stool, which is specific to detect lower gastrointestinal bleeding. Its user-friendly platform with resultant higher screening uptake, higher sensitivity for early-stage CRC, and high-throughput features make FIT the most popular test in population CRC screening worldwide. In 2004, Taiwanese government launched a nationwide screening program after a successful pilot program, and FIT is offered biennially to individuals aged 50 to 69 (extended to 75 in 2013). The screening (coverage) rate of this screening was 21.4% and the repeat screening rate was 28.3% in the inaugural 5 years (2004-2009) but improved to 56.6% and 52.3% in 2014. A recent analysis from the program has demonstrated that CRC mortality and incidence of advanced-stage CRC have reduced by 35% and 29%, respectively, when comparing those who did and did not participate in FIT screening.
Nevertheless, there are some challenges and obstacles that needed to be overcome. First, participation in the program is still satisfactory. How to increase the awareness of the public, especially the hard-to-reach population, and to minimize inequality in screening is an urgent task. Moreover, some individuals are not compliant with colonoscopy after a positive FIT, which largely affects the effectiveness of the screening. Both the government and professional societies should elaborate on increasing the awareness toward CRC by the public. Second, if we look at the results of Taiwan CRC Screening Program, the effectiveness of FIT screening on reducing CRC death and incidence of advanced-stage CRC was consistently lower in the proximal colon than in the distal colon. This arouses the attention of interval cancers, with maybe secondary to insufficient performance of FIT for proximal colon and inadequate colonoscopy quality. There is also a discrepancy in the quality of colonoscopy among different units and individual endoscopists. Continuous effort in improving colonoscopy quality and clinical audit is indispensable. Third, young-onset CRC is rapidly increasing not only in the West but also in Asian countries. Adjusting the age of starting screening may need to be revised and more relevant researches are highly demanded. Finally, whether novel technologies such as artificial intelligence would be able to contribute to improving the effectiveness of screening is intriguing. The screening community should not hesitate to embrace them.
Last but not least, CRC screening, one of the most effective interventions to reduce the death from the most prevalent cancer in our country, has been underfunded for a long time by the government. Long-lasting financial support for this program is necessary for its sustainable development and success. All of these problems need to be remedied via collaboration between the screening organizer, screening distributor, and professional societies.
References
- GLOBOCAN. Estimated number of Colorectal cancer new cases in 2018, worldwide, both sexes, all ages Lyon, France: IARC; 2018.
- Rabeneck L, Chiu HM, Senore C. International Perspective on the Burden of Colorectal Cancer and Public Health Effects. Gastroenterology. 2020;158:447-452.
- Onyoh EF et al. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr Gastroenterol Rep. 2019;21:36. doi: 10.1007/s11894-019-0703-8.
- Chiu HM et al. Long-term effectiveness of faecal immunochemical test screening for proximal and distal colorectal cancers. Gut. 2021 Jan 25 (ePub):gutjnl-2020-322545. doi: 10.1136/gutjnl-2020-322545.
- Wang YW et al. Current status and future challenge of population-based organized colorectal cancer screening: Lesson from the first decade of Taiwanese program. J Formos Med Assoc. 2018;117:358-364.
- Lee YC et al. Effects of screening and universal healthcare on long-term colorectal cancer mortality. Int J Epidemiol. 2018 ;48:538-548.
- Chiang TH et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology. 2014;147:1317-26.
- Chiu SY et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017;66:293-300.
- Lee YC et al. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Nat Cancer Inst. 2017;109.
- Cheng SY et al. Factors affecting compliance with confirmatory colonoscopy after a positive fecal immunochemical test in a national colorectal screening program. Cancer. 2018;124:907-915.
- Peng SM et al. Faecal immunochemical test after negative colonoscopy may reduce the risk of incident colorectal cancer in a population-based screening programme. Gut. 2021;70:1318-1324.
- Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, Kyaw MH. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol. 2019;114:322-329.
- Chiu HM et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121:3221-9.
- Matthew Fulco. Colorectal Cancer Remains Prevalent in Taiwan. Taiwan Business TOPICS. American Chamber of Commerce in Taiwan (AmCham Taiwan). https://topics.amcham.com.tw/2021/08/colorectal-cancer-prevalent-taiwan/

Figure 1: Professor Chiu in the 33rd Hong Kong International Workshop on Therapeutic Endoscopy
Figure 2: Professor Chiu in the 33rd Hong Kong International Workshop on Therapeutic Endoscopy (Left: prof Yutaka Saito, Right: prof Noriya Uedo)
Figure 3: Fecal immunochemical test (FIT) - cheap but big effect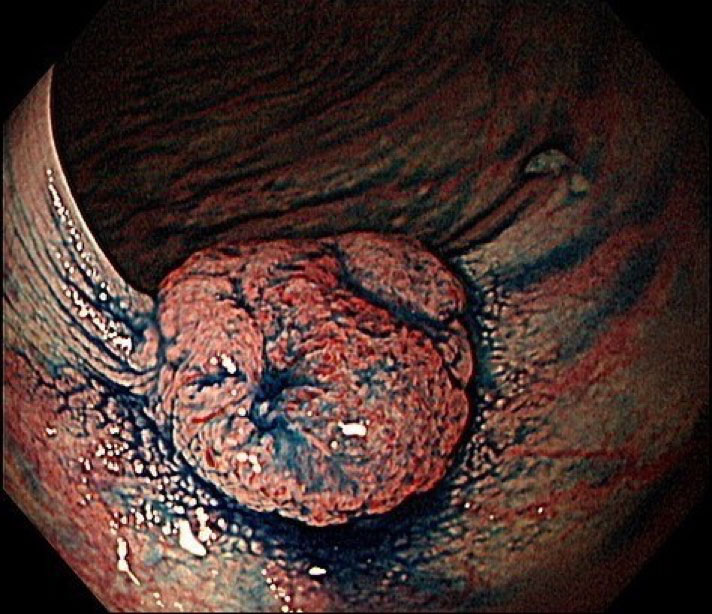
Figure 4: Screening detected early (T1) colon cancer -
 Motility examination of GI tractChing-Liang Lu M.D.
Motility examination of GI tractChing-Liang Lu M.D.Endoscopy Center for Diagnosis and Treatment, Taipei Veterans General Hospital; Institute of Brain Science, National Yang Ming Chiao Tung University, Taipei, Taiwan
Gastrointestinal (GI) motility disorders are very common and contribute to around half of the patients in the outpatient clinic of our daily practice. Motility of the GI tract is an important function to facilitate ingested food for digestion, nutrients absorption, and waste elimination. And the GI motility is the results from the interplay among the nervous system, the endocrine system, smooth muscle cells, interstitial cells of Cajal, secretory mucosal cells, the immune system, and even gut flora. Abnormal GI motility, or dysmotility, may lead to nonspecific GI symptoms.
In the past, the nonspecific nature of these GI symptoms, the absence of an organic lesions on clinical investigations (such as blood tests, routine radiology, and even endoscopy) would make management very challenging for both the patients and physicians. In recent years, advances technologies such as, high resolution manometry (HRM), have gained great progress in the understanding of these GI motility diseases. Many motility tests are widely used to assess a multitude of GI motility diseases along the digestive tract, including pH/impedance test, 96 hour Bravo pH test, esophageal high resolution esophageal manometry (HRIM), gastric emptying scintigraphy, antroduodenal manometry, colonic manometry, and anorectal manometry. In addition, hydrogen breath test for small bowel bacterial growth (SIBO), Sitzmarks test, wireless motility capsule, Endoflip, timed barium swallow, and defecography are also attributed to motility tests to identify the potential causes to explain the chronic GI symptoms. Despite of these facts, it is still important to exclude an anatomic lesion or other organic etiologies for the chronic symptoms before proceeding with motility evaluation is extremely important.
Among the mentioned tests, esophageal HRIM and pH-impedance tests are the most commonly used examinations in Taiwan, which modalities are recently get reimbursement from Taiwan National Health Insurance. The esophageal HRIM test used a high-resolution manometry catheter, bearing 36 pressure transducers that are spaced out in 1 cm intervals. Pressure measurements are obtained and analyzed by standardized metrics including the integrated relaxation pressure, contractile front velocity, distal contractile integral, distal latency, and peristaltic break size. The results are separated into 2 main categories of motility disorders, i.e. disorders of EGJ outflow and disorders of peristalsis, by the updated Chicago classification, now in its 4.0 version. High-resolution anorectal manometry (HRAM) is also a new technique to measure intraluminal pressure activity in anorectum using a series of closely spaced pressure sensors. HRAM can provide a new perspective on the pathophysiologic mechanisms of disordered defecation through the measurement of anorectal function. Just like the mode of Chicago process in the classification of esophageal motor disorder, a London classification from the efforts of the International Anorectal Physiology Working Group (IAPWG) has been developed to apply a standardized protocol for setting up the indications, application, and clinical interpretation of anorectal motor function. Ambulatory pH with or without simultaneous multiple intraluminal impedance (MII) measurements are suggested to establish the diagnosis of GERD by current guidelines. The sensitivity of the MII-pH examination is optimal only after stopping PPI for at least 7 days before the tests. For the MII-pH tests, the impedance is able to detect all reflux events, including liquid (acidic or alkaline), gas, or mixed contents. Additionally, impedance measurements can detect the direction of movement of air/liquid through the esophagus and differentiate conditions such as swallowing, aerophagia and supragastric belching that can be associated or trigger the symptoms. For the patients with catheter intolerance in MII-pH (around ~10-20 % of patients), wireless pH monitoring (Bravo system, Medtronic) provides an alternative way tolerated by most patients. Another advantage of this wireless system is that this approach can have a prolonged pH monitoring for up to 96 hours’ monitoring, which improves the diagnostic accuracy of GERD by better establishment of the association between acid reflux and symptoms.
Advance technology can now provide more meaningful assessment for GI motility and its function. Through the accurate and objective measurements from these technologies, it would enable us to reach definitive diagnoses in GI motility disorders and chose the corresponding treatment in clinical practice. Nevertheless, under some situations, we can only identify the potential causes for the chronic non-specific symptoms without a definite way to treat in these patients with functional GI disorders. However, even when no specific treatment can be provided based on the results of these motility tests, we can still give a clear explanation of the causes of symptoms for these patients, which measurement could also be a therapeutic option and satisfied the patients. Future researches are mandatory to understand the pathological basis of GI motility disorders to determine the phenotype of patients with GI dysmotility. Subsequent development of novel tests and treatments for these patients with abnormal motility is also important. Furthermore, many of the mentioned motility tests are still not available in Taiwan. For the benefits of our patients and physicians, we should try our best to introduce these ‘new’ (actually ‘old’ for the World) modalities to Taiwan.
References
- Carrington EV, Heinrich H, Knowles CH, et al. The international anorectal physiology working group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil 2020;32:e13679.
- Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil. 2021;33(1):e14058.
- Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351-1362

-
 Frontiers in Taiwan GI Endoscopy : Interventional EUSYu-Ting Kuo, MD MSc
Frontiers in Taiwan GI Endoscopy : Interventional EUSYu-Ting Kuo, MD MScDivision of Endoscopy, Department of Integrated Diagnostics & Therapeutics, National Taiwan University College of Medicine, Taipei, Taiwan
In the last years, endoscopic ultrasound (EUS) has evolved from a purely diagnostic technique to a more and more complex interventional procedure. Recently, there have been significant advancements in interventional EUS procedures, including EUS-guided drainage procedures, EUS-guided anastomosis and tumor ablations over the past two decades. The described procedures include celiac plexus neurolysis and block, ablation of pancreatic neoplasm, EUS-guided drainage of pancreatic fluid collections, biliary drainage, pancreatic duct drainage, gallbladder drainage, and gastroenterostomy. These procedures have been made possible with novel devices that can reduce the difficulties of the procedures and potentially reducing the risk of adverse events. The procedures are becoming standardized and evidence is accumulating demonstrating advantages of these procedures.
Actually, the concept of performing EUS-guided anastomosis between two target organs is not new. In 1993, Grimm et al. first described EUS-guided drainage of a pancreatic pseudocyst. Thereafter, Giovannini et al. described EUS-guided cholechoduodenostomy in 2001. EUS-guided pancreaticogastrostomy and cholecystoduodenostomy were then described in 2002 and 2007, respectively using similar techniques. However, these techniques did not gain popularity due to several reasons. Firstly, a therapeutic echoendoscope was not available, and the step for stent insertion required an exchange to a duodenoscope. Secondly, dedicated EUS devices for performing the procedures were not available, and tools used were frequently borrowed from endoscopic retrograde cholangiopancreatography (ERCP) increasing the difficulties of the procedures. In addition, the margin of error for these procedures is small, meaning that if an adverse event like stent misdeployment occurs, the ability to endoscopically salvage the procedures is low. Unlike surgical anastomosis, the integrity of the anastomosis in EUS-guided anastomosis is entirely dependent on the stent. Hence, the availability of a reliable stent is paramount.
More recently, new metal stents specifically designed for transluminal drainage, namely lumen-apposing metal stents (LAMS), have been made available for EUS-guided procedures in 2011. These stents share several design characteristics as following: large diameters, short in length, and possess flanges on the two ends of the stent to hold two organs together and to prevent stent migration. Furthermore, the performance of EUS-guided anastomosis is a multi-stepped procedure. This multi-stepped procedure increases the risk of encountering mishaps during the procedure, which may result in severe adverse events. Recently, several electrocautery enhanced single-step delivery systems have become available and it allows direct access of the delivery system to the target lumen, has subsequently simplified the classic multi-step procedure of EUS-guided drainages. Thus, this significantly reduces the procedure time, difficulty, and also the risk of adverse events.
EUS-guided drainage of pancreatic fluid collections is now the preferred approach. Several randomized controlled trials over the last decade have demonstrated that in the minimally invasive treatment of acute necrotic collections and wall of necrosis (WON) yield better outcomes, shorter length of stay and overall lower cost of care than open surgical necrosectomy. In recent years, LAMSs have gained popularity in the management of pseudocysts and WON. While data for their superiority over plastic stents are conflicting, potential advantages of LAMS include a wider lumen to allow passage of necrotic tissue and the ease of performing endoscopic necrosectomy through the stent lumen.
EUS-guided biliary access and drainage (EUS-BD) have been shown in several randomized controlled trials and meta-analyses to have relatively high technical and clinical success rates (85% or higher for both) and a low, though not insignificant, risk of adverse events of up to 15%. Potential adverse events include bowel perforation, bleeding, bile leakage and peritonitis. However, the risk of pancreatitis is averted by performing EUS-BD. EUS-BD procedures require technical expertise in diagnostic and therapeutic EUS. Given the various approaches involved, it is important to be familiar with the various options and the associated unique technical aspects of the procedures. In patients with malignant biliary obstruction and failed ERCP, the major advantage of EUS-guided drainage is internalization of the stent thus minimizing the morbidity associated with long-term percutaneous drainage tubes and avoiding surgery in high-risk patients.
EUS-guided gallbladder drainage (EUS-GBD) in patients with cholecystitis may be considered in those unable to undergo definitive management with cholecystectomy. EUS-GBD is associated with a higher technical and clinical success rate compared to percutaneous GBD (90-98% and 89-97% respectively). A recent international, randomized controlled trial compared EUS-GBD to percutaneous cholecystostomy and demonstrated high technical success rates (97.4% vs 100%, p=0.494) for both procedures but significantly lower adverse event rates for the EUS-GBD group. The EUS guided approach offers an alternative to ERCP assisted transpapillary drainage with a relatively small caliber plastic stent or percutaneous cholecystostomy and its drain-related comorbidities.
EUS guided pancreatic duct access has been described in patients in whom retrograde pancreatic duct access is not successful at ERCP, not possible due to altered anatomy (i.e. inability to reach or visualize the pancreatic duct orifice after surgery) and in the setting of disconnected pancreatic duct syndrome. Unlike other EUS-guided drainage and access procedures, there has been limited improvement in technology to make EUS-guided pancreatic access easier or safer. Therefore, there remains a significant need for the development of EUS guided devices that enable easy access and drainage of the pancreatic duct in a safe and reliable manner.
EUS-guided enteral anastomoses have become possible with the introduction of LAMS, especially cautery assisted LAMS. These stents permit creation of new entero-enteric anastomoses in malignant and benign gastric outlet obstruction (EUS gastrojejunostomy, EUS-GJ), drainage of obstructed small bowel limbs after surgery and creation of fistulae after Roux-en-Y gastric bypass to facilitate ERCP. Potential advantages of EUS-GJ include immediate post-procedure reintroduction of oral intake and administration of chemotherapy in patients with malignant obstruction. Although, prospective comparative data are lacking, EUS-GJ has been shown to have similar efficacy and safety compared to surgical GJ in a small retrospective study.
Back to the developmental history of interventional EUS in National Taiwan University Hospital, EUS-guided fine needle aspiration was first described in 2000. Then, EUS-guided pseudocyst drainage, EUS-guided nerve block and EUS-guided choledochoduodenostomy were all described in 2007. In 2011, EUS-guided rendezvous pancreatic stenting was first described. We have started to perform EUS-guided ethanol injection and EUS-guided radiofrequency ablation for pancreatic solid lesions in 2013 and 2019, respectively. Thereafter, EUS-guided hepaticogastrostomy, EUS-guided gallbladder drainage and EUS-guided gastrojejunostomy have been performed since 2020 after LAMS was approved by TFDA.
To this date, totally 85 patients in NTUH receiving EUS-guided drainage procedures and EUS-guided anastomosis. Both of overall technical and clinical success rate were 92.6% and adverse events rate was 7.4%. Although our results were not inferior to reported data from the renowned centers in the world, there are still a number of limitations in Taiwan. First, these EUS procedures are still in evolution and the devices used are not uniformly available. Hence, they should be adopted after consideration of device availability, institutional practices and endoscopist’s preferences. Second, the use of EUS-specific metal stents and devices needs to be justified due to its higher cost not covered by our National Health Insurance. Finally, mostly interventional EUS procedures were alternative choice to current standard treatments. Expert multidisciplinary discussion with radiologists, surgeons and oncologists is needed before performing interventional EUS procedures.
References
- Teoh AYB, Dhir V, Kida M et al. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut 2018.
- Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy 2011; 43: 337–42.
- Han SY, Kim SO, So H, et al. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci Rep. 2019; 9(1):16551
- Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020.
- DeWitt JM, Arain M, Chang KJ, et al. Interventional Endoscopic Ultrasound: Current Status and Future Directions. Clin Gastroenterol Hepatol. 2021;19(1):24-40
Figure1. Effective teamwork is an important aspect of interventional EUS's success.
Figure 2. A) EUS-guided gallbladder drainage. B) EUS-guided choledochogastrostomy. C) EUS-guided hepaticogastrostomy. D) EUS-guided gastrojejunostomy. -

Coronavirus disease-2019 (COVID-19) has become global pandemic since February 2020. Taiwan have adopted stringent measures to protect populations from spread of infection. Despite these measures, the number of infected people is growing exponentially with a significant number of patients developing severe acute respiratory syndrome. Transmission can occur in both symptomatic and asymptomatic individuals. Endoscopy units face significant risk for respiratory diseases that can be spread via an airborne route. Endoscopy department staffs are at a significant risk for respiratory diseases that can be transmitted via an airborne route.
To prevent COVID-19 transmission in endoscopy centers, we summarize and recommend the following infection prevention and control workflow in endoscopy units (Figure 1) according to Digestive Endoscopy Society of Taiwan and standard practice in current gastroenterology guidelines.
The purpose of this section specifically focused on pre-screening of patients (Table 1, 2), endoscopy center check-in process, personal protection equipment (Figure 2), endoscope reprocessing, and endoscopy staff training. It is anticipated that physician and staffs resume clinical practice, preventing further dissemination of COVID-19 infection, while ensuring the safety in in gastrointestinal endoscopy units.
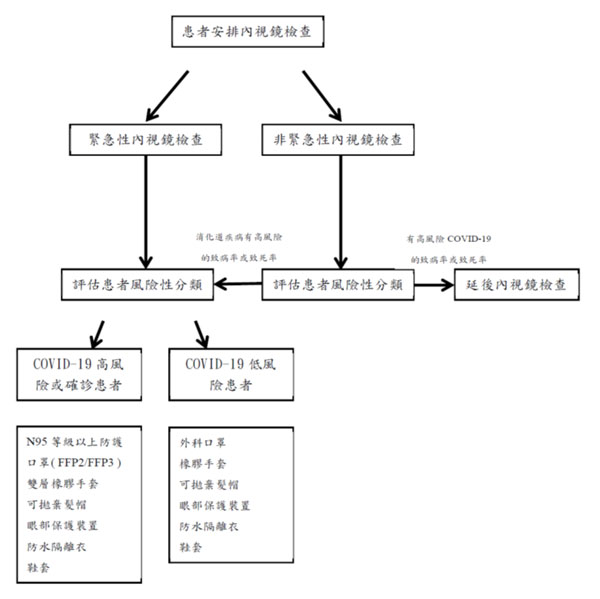
Figure 1. COVID-19 流行時, 內視鏡單位建議處置流程圖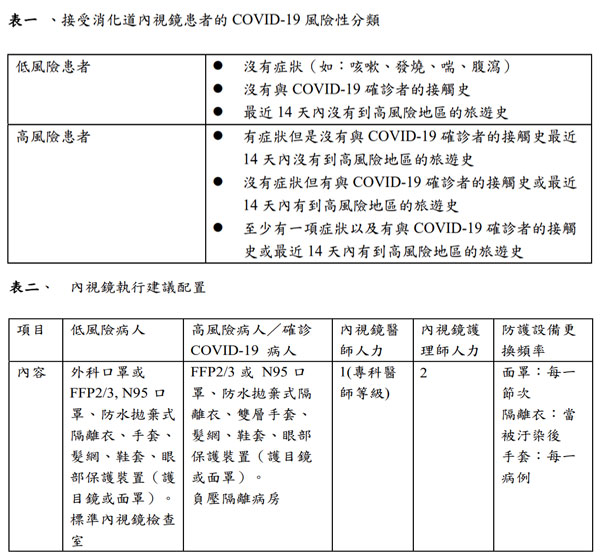
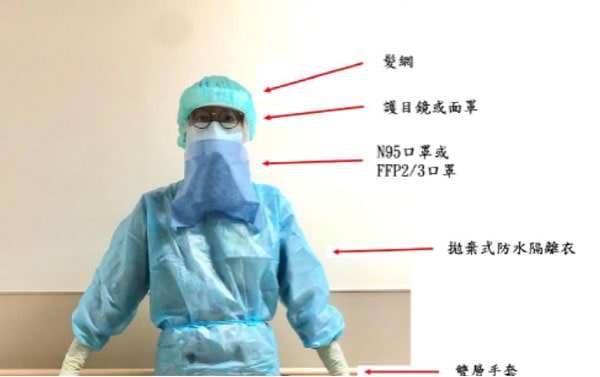
Figure 2. 個人防護裝備,包含手套、髮網、鞋套、眼部保護裝置(護目鏡或面罩)、防水拋棄式隔離衣以及呼吸保護設備(口罩)。高危險或感染案例使用高過濾率的口罩(FFP2/3等級, N95)。 -
 AI in GI endoscopyPeng-Jen Chen
AI in GI endoscopyPeng-Jen ChenAssociate Professor, Division of Gastroenterology, Tri-Service General Hospital
Artificial intelligence (AI), machine learning (ML), and deep learning (DL)
Artificial intelligence (AI) developed rapidly in these recent years, and it has been applied in our daily lives, such as forecasting weather, self-driving cars, and predicting the behavior of customers for web-based applications. DL and ML are defined as the ability of AI to learn representations of data.1 Since the 1980s, ML has been performed to construct a mathematical model and predict outcomes based on input data. Recently, as a subset of ML, DL based on artificial neural networks has become the most popular methods of AI.2 A deep neural network (DNN) is an artificial neural network (ANN) with multiple layers between the input and output layers. The adjective "deep" in deep learning refers to the use of multiple layers in the network. Since the 2010s, advances in both deep learning algorithms and computer hardware have led to more efficient methods for training deep neural networks that contain several complicated non-linear hidden layers. Graphic processing units (GPUs) with AI-specific computational ability has displaced CPUs as the major core component to train huge-scale DNNs.
Deep learning has the ability to feed on large amounts of data, train, and improve the DNN model. Deep learning holds great promise in clinical medicine. It is suitable for analysis of complex or multi-dimensional medical images using the large-scale and multi-layers of convolutional neural networks (CNN).3 Convolutional and pooling layers that extract distinct features and fully connected layers can make a final classification, and CNNs have demonstrated excellent performance in image classification. Artificial intelligence have great potential in gastroenterology. Several imaging modalities of radiology, endoscopy and pathology, have been used for evaluation of the digestive tract and gastrointestinal (GI) tumors. These images can be analyzed using machine learning approaches. In the fields of gastroenterology and hepatology, AI could play a significant role in the diagnosis and treatment of GI diseases.
AI in GI endoscopy
Because of the heterogeneous expertise levels of endoscopists, time-consuming procedures, and increasing workloads, evaluating endoscopic images visually to detect and diagnose GI lesions are largely based on expertise and experience of endoscopists.4 CNN, a subtype of ANN, are able to analyze large amount of parameters of image data in an efficient way. By taking in large quantities of image data sets, CNN can be trained to optimize the model to identify or detect new similar objects from other images. Streaming signals, coming from camera or videos, analyzed real-time by AI is referred to computer vision. Applying methods of both CNNs and computer vision can be powerful in endoscope, such as computer-aided diagnosis (CADx) and computer-aided detection (CADe). CADx can classify endoscopic images and CADe can detect specific objects during endoscopic examinations. Numerous endoscopic studies of computer-aided diagnosis and computer-aided detection have been published in these recent years.5 AI is able to be applied in endoscopy, such as identification of esophageal and gastric neoplasia in esophagogastroduodenoscopy (EGD), detection of gastrointestinal bleeding in wireless capsule endoscopy (WCE), and polyp detection and characterization in colonoscopy…etc.
AI in Colonoscopy
Colorectal cancer (CRC) ranks third in cancer mortality and first in cancer incidence in Taiwan. About 10,000 people suffer from CRC every year. Colonoscopy is indicated for screening and surveillance of CRC, the evaluation of lower digestive tract symptoms, and endoscopic therapy for early colorectal neoplasms. The number of colonoscopy examinations paid by the self-financed and health insurance is nearly 100,000. Most CRCs arise from preexisting adenomatous polyps, and, removal of adenomas can reduce the incidence of CRCs and thus reduce its mortality. Adenoma detection rate (ADR) is inversely proportional to the risk of CRCs. An endoscopist’s ADR has been found to correlate directly the endoscopist’s rate for interval CRCs. However, ADR may vary depending on the doctor performing the examination, and this difference may be as high as 20%.
AI-assisted colonoscopy has been developed for polyp detection and characterization, and predicting the prognosis of CRC. Polyp characterization with magnifying endoscopic images is useful for identifying pit or vascular patterns to improve performance. AI tools with narrow-band imaging or endoscopic videos can be used to differentiate diminutive hyperplastic polyps and adenomas with high accuracy. Diminutive polyps (≤ 5 mm) may also be identified during colonoscopy with CADe.6
The AI status in Tri-Service General Hospital
The medical–engineering collaborative project between Tri-Service General Hospital and the Computer Science Department of National Chiao Tung University, initiated in 2016, resulted in the image classification model.7 The algorithm of the retraining model using TensorFlow, comprises four steps: (i) image collection, (ii) model learning, (iii) creation of a classifier, and (iv) diagnostic output. The initial computer-aided analysis system can differentiate between diminutive (< 5 mm) neoplastic polyps and hyperplastic polyps in images of full magnification.
Later, Tri-Service General Hospital initiated industry-Academic Cooperation with Taiwan Innovative Medical Solutions (TIMS), and developed a real-time computer-aided detection system during colonoscopy. (Figure 1, 2) The project collected a large number of colonoscopy digital images and digital videos for image analysis, classification and labeling, and used deep neural networks for detection of polyps and image coordinate regression to establish GI-CAD, a new artificial intelligence real-time detection system for colorectal polyps. GI-CAD might assist endoscopists to increase the ADR and reduce the visual fatigue caused by performing colonoscopy, so as to improve the efficiency of colonoscopy procedures and medical quality. GI-CAD is promising to be applied in clinical practice, and contribute to the prevention of CRCs.
Obstacles to implementation of AI technologies in GI endoscopy include software-hardware integration, the unclear regulations of the government, and clinical trials prior to clinical use. Although the progression of CADx and CADe, endoscopists should be aware of their limitations, such as risks of misdiagnosis, the black-box nature of AI technologies, and the quality of database for AI model development. Developers and users do not know the details about how computers output the conclusion, and AI models may potentially cause errors. If misdiagnosis occurs during AI application, the operator and the company providing the AI system should take the responsibility in both ethical and legal aspects. The regulatory approval is necessary before clinical practice.
- Yu C, Helwig EJ. The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif Intell Rev 2021:1-21.
- Song YQ, Mao XL, Zhou XB, et al. Use of Artificial Intelligence to Improve the Quality Control of Gastrointestinal Endoscopy. Front Med (Lausanne) 2021;8:709347.
- Jha D, Ali S, Tomar NK, et al. Real-Time Polyp Detection, Localization and Segmentation in Colonoscopy Using Deep Learning. IEEE Access 2021;9:40496-40510.
- Cao JS, Lu ZY, Chen MY, et al. Artificial intelligence in gastroenterology and hepatology: Status and challenges. World J Gastroenterol 2021;27:1664-1690.
- Berbis MA, Aneiros-Fernandez J, Mendoza Olivares FJ, et al. Role of artificial intelligence in multidisciplinary imaging diagnosis of gastrointestinal diseases. World J Gastroenterol 2021;27:4395-4412.
- Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc 2021;93:77-85 e6.
- Chen PJ, Lin MC, Lai MJ, et al. Accurate Classification of Diminutive Colorectal Polyps Using Computer-Aided Analysis. Gastroenterology 2018;154:568-575.
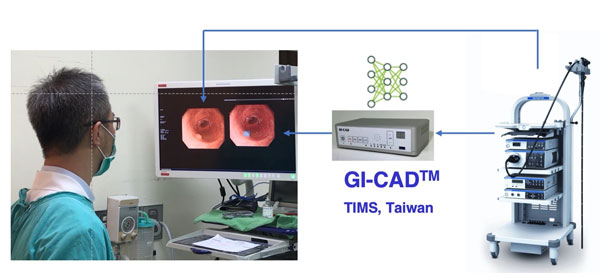
Figure 1. The real-time computer-aided detection system in Tri-Service General Hospital.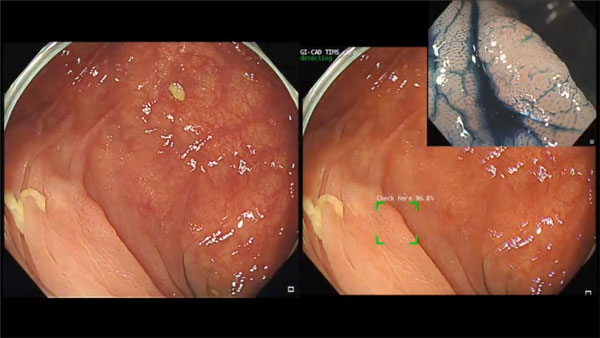
Figure 2. One flat adenoma is detected by GI-CAD. -
 Frontiers in Taiwan GI Endoscopy:Inflammatory Bowel DiseasesShu Chen Wei MD, PhD, AGAF
Frontiers in Taiwan GI Endoscopy:Inflammatory Bowel DiseasesShu Chen Wei MD, PhD, AGAFDepartment of Internal Medicine,National Taiwan University Hospital and College of Medicine, Taipei, Taiwan
During the past two decades, the incidence and prevalence of inflammatory bowel diseases (IBDs), which include the ulcerative colitis (UC) and Crohn’ disease (CD), increased significantly in Taiwan. In contrast to the old days, when IBDs belong to rare disease, the disease awareness has been raised dramatically in recent years. This is a good thing to decrease under-diagnosis. However, we also starting to face another problem, the over- or mis- diagnosis of IBD. Therefore, how to make a correct diagnosis of IBD is an extremely important issue. Since the treatment between IBDs and non-IBDs (especially the infectious colitis) differs quite a lot, and sometimes, even in the opposite direction. Early and correct diagnosis is the first important step to provide appropriate treatment, which can lead to decrease the disease related complications (such as stenosis, perforation) and morbidity (hospitalization and surgery). With the importance in clinical practice, this year, during the annual activity of DEST (Digestive Endoscopy Society of Taiwan), we will invite four distinguished speakers to share their fantastic experience about the differential diagnosis of IBD and its mimickers, the endoscopic management of IBD related complications, and the optimization of performing scope in IBD patients (focusing on standardization of the report form and cancer surveillance).
“Endoscopic diagnosis of IBD and its mimickers”: Endoscopic appearance, including the inflammation/erosion/ulcer morphology, location, distribution, all are important clues for correct diagnosis of IBD. When reading with histology findings via biopsy, most IBD patients could be diagnosed. However, history taking and stool culture for excluding other infectious etiology are extremely crucial preludes for differential diagnosis. For some patients who cannot be diagnosed for sure, treatment and monitor the response will be the possible solution. For whom the treatment response is not satisfied, re-evaluation is highly recommended.
“Endoscopic management of IBD related complications”: For CD, stenosis is a common complication. When the inflammation has been under control but the stenosis persisted due to fibrosis and the patients start to having the obstructive symptoms, endoscopy can help in managing the stenosis by balloon dilatation. However, balloon dilatation still carries the risk of bleeding and perforation. Therefore, we have to choose the appropriate condition to perform the balloon dilatation (no ulcer around, stenosis less than 3cm, number less than 3 to 5 stenosis, and could be approached by endoscopy).
“Optimizing the quality of endoscopy in IBD: focus on surveillance”: As we mentioned earlier, endoscopy is an important tool for diagnosing IBD. The first endoscopy provides the most important information. A very important tip for diagnosing IBD is to have a complete ileo-colonoscopy, especially for those suspecting CD. Colitis associated colorectal cancer is a known long-term complication of under controlled UC. Therefore, for UC patients who have extensive inflammation, or long history of UC (more than 8 years for extensive type, 12 years for left side colon type), cancer surveillance is indicated. Nowadays, targeting biopsy after image enhancement, either by dye or NBI, is more favored than random biopsy.
“Optimizing the quality of endoscopy in IBD: focus on endoscopic report”: A standardized description of the inflammation, the size of ulcer, the location and distribution of ulcers, can provide the diagnosis information. This is also important for treatment response comparison.
In summary, we need to update our knowledge for IBDs, since these diseases become more common and would be even common in the foreseen future. Our experts for IBD in Taiwan will gather together to share their experience in this annul meeting. They previously have published related articles that I have listed as below for your reference.
- Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017 Jul; 15(3): 266–284.
- Management of Crohn's disease in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017 Jul; 15(3): 285–310.
- eBook: Case Studies and Endoscopic Images in Inflammatory Bowel Disease and its Mimics. http://www.tsibd.org.tw/news.php?index=81&c1=2
